Welcome to the intriguing world of organic chemistry, where every molecule tells a story. Today, we’re diving into the fascinating realm of 2 2-dimethylcyclohexanol with deuterium. This compound is not just another name on your periodic table; it’s a key player in many chemical reactions and has unique properties that set it apart from its counterparts. With its carefully structured molecular design, this alcohol opens doors to innovative applications and research possibilities. Join us as we unravel what makes 2 2-dimethylcyclohexanol with deuterium so special and explore its significance in the broader landscape of organic synthesis!
Overview of 2 2-Dimethylcyclohexanol with Deuterium
2 2-dimethylcyclohexanol with deuterium is an interesting organic compound that belongs to the cyclohexanol family. Its structure features a cyclohexane ring with two methyl groups attached at the second carbon position, making it a dimethyl derivative. This unique arrangement gives it distinct properties compared to other alcohols.
The molecular formula for 2 2-dimethylcyclohexanol with deuterium is C10H20O, and its presence plays a significant role in various chemical reactions. With its hydroxyl group (-OH) contributing to polarity, this compound can interact effectively with other molecules.
This compound finds applications in synthetic chemistry due to its versatile reactivity. Researchers often explore its behavior under different conditions, revealing insights into reaction mechanisms and pathways that may involve deuterium labeling for further study.
Importance of Deuterium in Chemical Reactions
Deuterium, a stable isotope of hydrogen, plays a crucial role in various chemical reactions. Its unique properties allow chemists to trace reaction pathways and mechanisms effectively. By replacing regular hydrogen atoms with deuterium, researchers can gain valuable insights into molecular behavior.
In kinetic studies, deuterated compounds exhibit different rates of reaction compared to their non-deuterated counterparts. This difference helps scientists understand how specific bonds break and form during chemical transformations. It’s a powerful tool for unraveling complex organic reactions.
Moreover, the incorporation of deuterium into molecules enhances stability in certain conditions. This stability is vital in drug design and development as it influences the pharmacokinetics of therapeutic agents. Deuteration opens new avenues for innovation within organic chemistry and beyond.
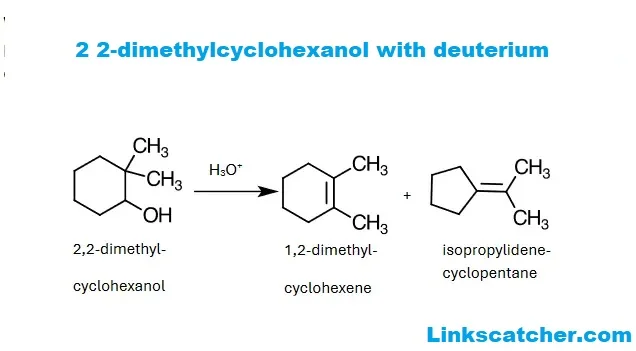
Acid-Catalyzed Dehydration of 2 2-Dimethylcyclohexanol with Deuterium
Acid-catalyzed dehydration of 2 2-dimethylcyclohexanol with deuterium is a fascinating process that transforms an alcohol into an alkene. In this reaction, sulfuric acid or phosphoric acid acts as the catalyst. The presence of these acids facilitates the removal of water from the hydroxyl group.
When heated, the alcohol undergoes protonation, making it more susceptible to losing a water molecule. This step is crucial as it leads to carbonation formation. Depending on stability and streaks, different pathways may emerge during this transformation.
The result is typically a mixture of alkenes formed through elimination reactions. These products can further react under different conditions, showcasing their versatility in organic synthesis and applications in complex molecular constructions.
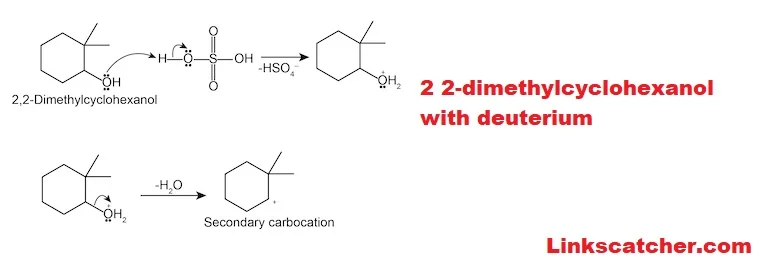
Mechanism of acid-catalyzed dehydration
The mechanism of acid-catalyzed dehydration for 2 2-dimethylcyclohexanol with deuterium is intriguing. It begins when the alcohol group protonates, forming a more reactive oxonium ion. This step increases the leaving ability of water molecules, making them easier to detach from the substrate.
Next, as water leaves, a carbonation intermediate forms at one of the carbon atoms where hydrogen was initially present. The stability of this carbonation significantly influences reaction rates and outcomes throughout this process.
A neighboring hydrogen atom migrates to stabilize the resulting positive charge on that carbon atom. A double bond then forms between these two carbon atoms, yielding an alkene product while completing the dehydration cycle in an efficient manner. Understanding this intricate mechanism helps chemists design better reactions using 2 2-dimethylcyclohexanol with deuterium.
Comparison to 2 2-dimethylcyclohexanol with deuterium
2 2-dimethylcyclohexanol with deuterium and 2 3-dimethylcyclohexanol may sound similar, but their structural differences lead to distinct chemical behavior. The positioning of the methyl groups alters steric hindrance and influences reactivity in various reactions. This subtle change can significantly affect product yields.
When comparing their properties, 2 3-dimethylcyclohexanol tends to display different boiling points and solubility characteristics. These variations can impact how each compound is utilized in organic synthesis or other applications within chemistry.
Additionally, the acid-catalyzed dehydration pathways diverge between these two compounds due to their structural arrangement. Understanding these differences helps chemists choose the right compound for specific reactions, making it essential for effective experimentation in laboratories.
Structural formula of 2 2-dimethylcyclohexanol
The structural formula of 2 2-dimethylcyclohexanol with deuterium reveals its unique arrangement of atoms. It consists of a cyclohexane ring with two methyl groups attached to the second carbon atom. This positioning defines its distinct properties and reactivity in chemical processes.
Visualizing the structure, you’ll see six carbon atoms forming a hexagonal shape, with hydroxyl (-OH) functionality contributing to its classification as an alcohol. The presence of these substituents affects both physical and chemical characteristics.
Understanding this structural formula is crucial for chemists exploring organic reactions involving 2 2-dimethylcyclohexanol with deuterium. Its configuration influences how it interacts with other molecules, making it a key player in various synthetic pathways within organic chemistry.
Application of 2 2-Dimethylcyclohexanol with Deuterium
2 2-dimethylcyclohexanol with deuterium plays a vital role in organic chemistry. Its structural uniqueness and properties make it an essential compound for researchers and chemists alike.
This versatile alcohol is often utilized as a reagent or solvent, aiding in various chemical reactions. Its presence can influence the rate of reactions, especially those involving isotopic substitution. This property proves useful in tracing mechanisms within complex organic transformations.
Moreover, due to its stable structure, 2 2-dimethylcyclohexanol with deuterium serves as a model compound for studying stereo chemical effects and conformational analysis. Researchers frequently explore its interactions in both synthetic pathways and reaction kinetics.
The incorporation of deuterium adds another layer of intrigue to this compound’s applications. The distinct behavior during hydrogen-deuterium exchange helps scientists gain deeper insights into reaction mechanisms.
The multifaceted nature of 2 2-dimethylcyclohexanol with deuterium underscores its importance in advancing our understanding of organic chemistry principles while driving innovation across various research domains.